The SI-Cure Implant was conceived and designed to optimize the treatment of patients with SI joint pain by providing compression, fixation and fusion. We have more than 5-years of clinical experience using a straight-forward and clinically proven lateral technique. By focusing on fusion, the SI-Cure Implant offers patients the potential for great outcomes.
- Patented design provides graft contact through the length of the implant
- Features a Self-HarvestingTM design that collects allograft as the Implant is advanced, thus eliminating the need for bone graft.
- As the Implant is advanced the helical design pushes the bone into the open architecture of the implant allowing the Self-AdvancingTM autograft to backfill the length of the Implant.
Intraoperative flouro image demonstrating SI-Cure Implant self-harvesting fenestrations
Intraoperative flouro image demonstrating SI-Cure Implant placement
3-month postoperative CT image demonstrating bony ingrowth
SEE HOW SI-CURE WORKS
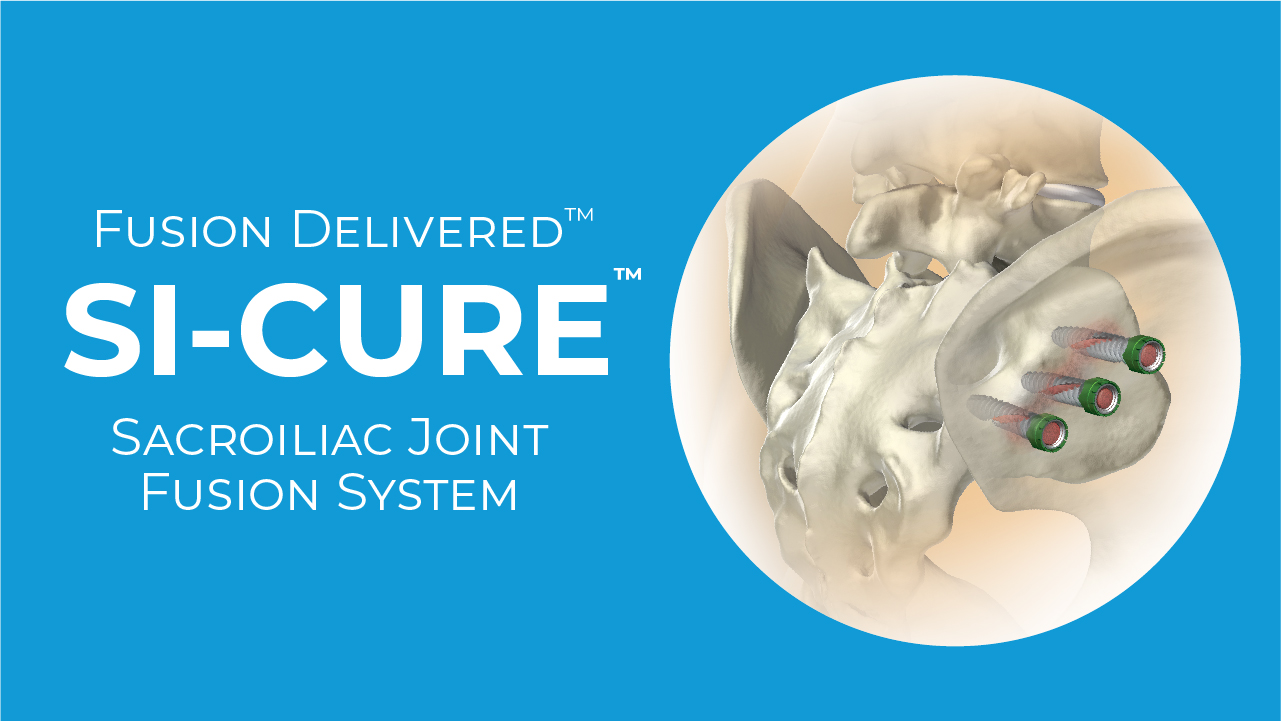
SI-Cure Sacroiliac Joint Fusion System
The SI-Cure Sacroiliac Joint Fusion System consists of Implants and a set of surgical instruments. The SI-Cure implants are cannulated, and fully threaded with double helix threads designed to be inserted into pre-drilled bone. The Implants are fabricated from medical grade titanium alloy, Ti-6Al-4V (ASTM F-136) and have a textured surface that encourages bony on-growth. The SI-Cure Implants come in various sizes to accommodate varying patient anatomy. Optional pivoting washers are included for each screw diameter to aid in conforming to patient anatomy.
Implant Specifications
- 9.5mm and 11mm Diameters
- Optional Load Distribution Cap
- Lengths 35-90mm
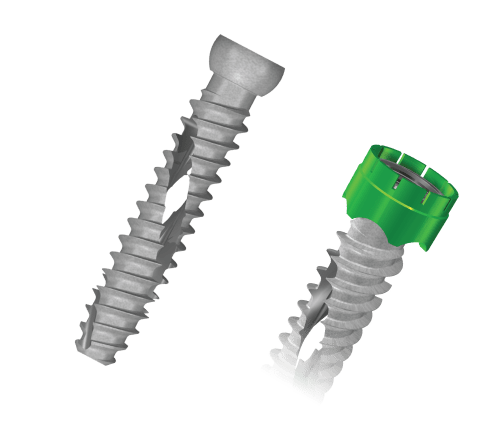
9.5mm Diameter Implants
9.5mm x 35mm, 900.095.35
9.5mm x 40mm, 900.095.40
9.5mm x 45mm, 900.095.45
9.5mm x 50mm, 900.095.50
9.5mm x 55mm, 900.095.55
9.5mm x 60mm, 900.095.60
9.5mm x 65mm, 900.095.65
9.5mm x 70mm, 900.095.70
9.5mm x 75mm, 900.095.75
9.5mm x 80mm, 900.095.80
9.5mm x 85mm, 900.095.85
9.5mm x 90mm, 900.095.90
9.5mm LOAD DISTRIBUTION CAP
9 .5mm, 900.095

11mm Diameter Implants
11mm x 35mm, 900.11.35
11mm x 40mm, 900.11.40
11mm x 45mm, 900.11.45
11mm x 50mm, 900.11.50
11mm x 55mm, 900.11.55
11mm x 60mm, 900.11.60
11mm x 65mm, 900.11.65
11mm x 70mm, 900.11.70
11mm x 75mm, 900.11.75
11mm x 80mm, 900.11.80
11mm x 85mm, 900.11.85
11mm x 90mm, 900.11.90
11mm LOAD DISTRIBUTION CAP
11mm, 900.11
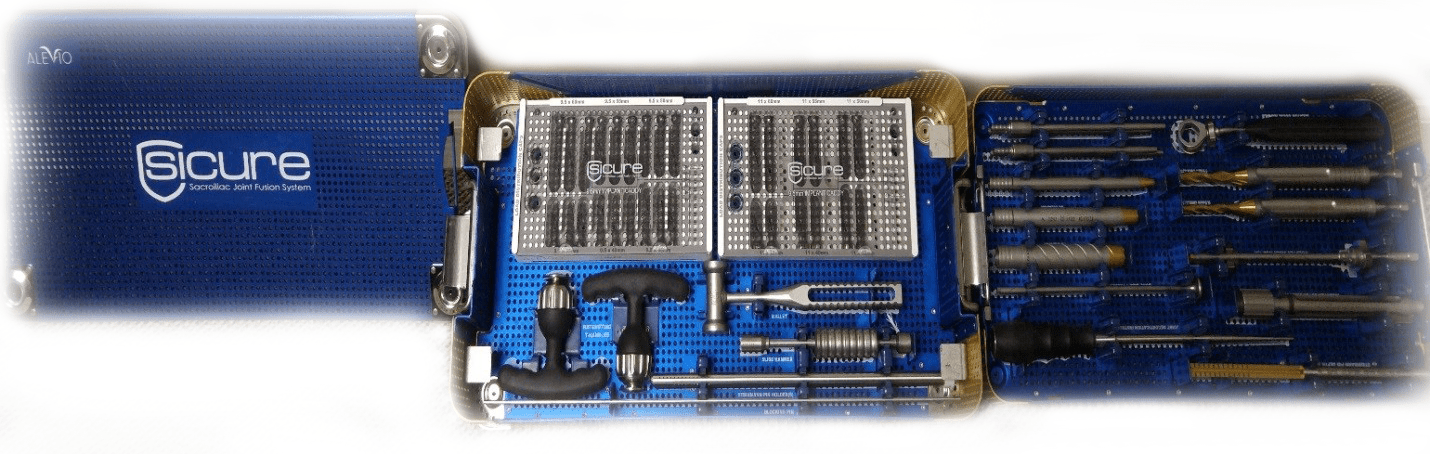
Surgical Instrument System
The SI-Cure Surgical Instrument System is comprised of various surgical instruments used to prepare the site to insert the implants.
All the instruments are made from surgical grade materials in the United States.
Indications for Use
The SI-Cure Sacroiliac Joint Fusion System is intended for sacroiliac fusion for the following conditions:
- Sacroiliac joint dysfunction that is a direct result of sacroiliac joint disruption and degenerative sacroiliitis. This includes conditions whose symptoms began during pregnancy or in the peripartum period and have persisted postpartum for more than 6 months.
- To augment immobilization and stabilization of the sacroiliac joint in skeletally mature patients undergoing sacropelvic fixation as part of a lumbar or thoracolumbar fusion.
- Acute, non-acute, and non-traumatic fractures involving the sacroiliac joint.
Contraindications
Contraindications for use of the SI-Cure Sacroiliac Joint Fusion System include,
but are not limited to:
- Infection
- Tumor
- Severe osteoporosis
- Mental or physical impairments that limit a patient’s ability to comply with necessary limitation of postoperative instructions
Refer to the SI-Cure Sacroiliac Joint Fusion System package insert document (FRM-002.4) for complete safety information including
- Warnings and Precautions
- Potential Adverse Events
- Care and Handling of Instruments
- Sterilization and Packaging

