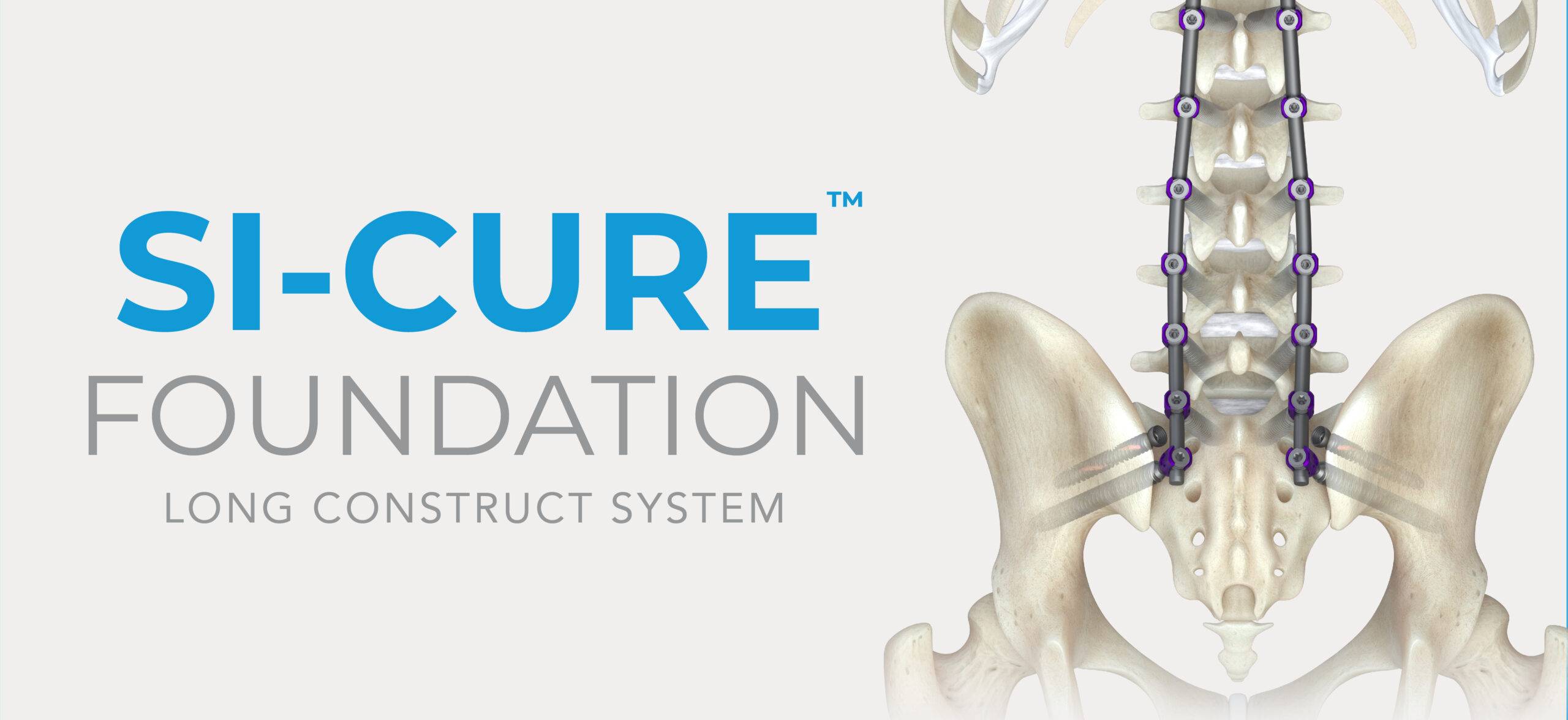
Studies have demonstrated a high incidence of SI joint pain in patients who have had a lumbar fusion.1 A retrospective study of patients who underwent lumbosacral fixation using SAI screws with at least 2 years of follow-up showed that loosening was observed in 50% of SAI screws. Furthermore, the bony fusion rate at L5–S was significantly lower in patients with SAI screw loosening than in those without screw loosening (65.0% vs. 93.3%, p=0.048).2
The SI-Cure Foundation™ Implant provides a proven option for your patients who are undergoing sacropelvic fixation as part of a lumbar or thoracolumbar fusion. The SI-Cure™ Implant was conceived and designed to optimize the treatment of patients with SI joint pain by providing compression, fixation and fusion. We have more than 5-years of clinical experience using a straight-forward and clinically proven technique. By focusing on fusion, the SI-Cure Implant offers patients the potential for great outcomes.